Abstract
Background
Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) characterised by the frequent presence of driver mutations in genes causing activation of JAK2 signalling pathways (JAK2, CALR and MPL). Additional mutations affecting epigenetic regulators and splicing machinery are common. Anaemia with RBC-transfusion-dependence is common in patients with advanced myelofibrosis and represents a major unmet need. The RESUME study assessed the rates of RBC-transfusion independence (TI) after therapy with Pomalidomide (POM) vs placebo in persons with MPN-associated myelofibrosis and RBC-transfusion dependence. 16% of patients in both the POM and placebo arms became TI.
Aims
The genetic landscape of strictly confirmed transfusion dependent MF is not fully characterised. Our aim was to analyse the genetics of transfusion-dependent myelofibrosis patients in the RESUME trial and correlate with clinical characteristics, outcome and therapy response.
Methods
DNA samples were available from 207 of 252 patients and analysed by targeted re-sequencing using a Fluidigm Access Array gene panel followed by next generation sequencing. The panel included JAK2, CALR, MPL, TET2, ASXL1, EZH2, DNMT3A, IDH1/2, CBL, IKZF1, U2AF1, CHEK2, TP53, SF3B1, SRSF2, SH2B3, BARD1, DAP3, HRAS, IRF4, KRAS, KIT, Mir662, NFE2, POLG, SCRIB, SETBP1, TCF12 and VPS45.
Results
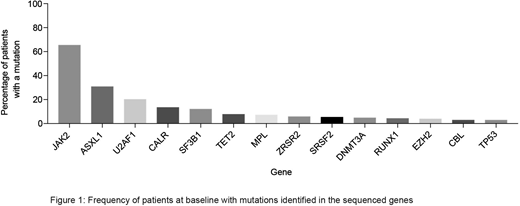
97% (95-99%) of subjects had a mutation in ≥1 targeted gene. 2 mutations were detected in 41% (34-48%) and ≥3 in 27% (21-33%). 7 had no detectable mutation. Mutations in JAK2V617F, CALR and MPL were identified in 66% (59-72%), 14% (8-19%) and 7% (4-11%) of subjects (Figure 1), with no driver mutation in 27 patients (13%; 9-18%) (triple-negative). 68% (61-74%) had additional non-driver mutations. 42% (35-48%) (N=86) had spliceosome mutations (U2AF1 [21%]; SF3B1 [11%]; SRSF2 [8%]; ZRSR2; [6%]). More spliceosome mutations were detected in men than women (47% [39-55%] vs 27% [15-40%]; p=0.009). Spliceosome mutations were mutually exclusive in 83 subjects and were less common in subjects with prior polycythaemia vera (17% [5-37%]) compared with prior essential thrombocythaemia (39% [22-58%]) and primary MF (46% [38-54%]; p=0.024). Mutations in epigenetic regulators (ASXL1, 28%; TET2, 8%; DNMT3A 5%; EZH2 4%) were detected at similar rates to those previously reported. High molecular risk (HMR) mutations (ASXL1, EZH2, IDH1/2, SRSF2) were detected in 36% [29-43%] of subjects. Only 10 of 105 subjects with an epigenetic regulator gene mutation had ≥1 related mutation. Subjects with JAK2V617F were significantly more likely than subjects with a CALR mutation to have: (1) ≥1 additional mutation (72% [64-79%] vs. 35% [18-54%], p=0.0001); (2) a spliceosome mutation (44% [36-53%] vs. 17% [6-36%], p=0.07), in particular a U2AF1 mutation (24% [17-32%] vs. 0%; p=0.004) and (3) a HMR mutation (38% [30-47%] vs. 21% [8-40%]; p=0.07). Survival at 1.5 years was 62% (55-67%) and was not significantly associated with the presence or number of mutations in this uniformly high-risk cohort. Survival in subjects without an SF3B1 mutation was better than those SF3B1-mutated (80% [56-91%]) vs. 59% [52-65%]; p=0.07). Driver mutation status did not influence the probability of achieving red blood cell (RBC) TI, regardless of therapy. Additional non-driver mutations were more often detected in those failing to achieve RBC-TI than those achieving RBC-TI (70% [63-77%] vs 56% [40-71%], p=0.07). Furthermore, those with additional non-driver mutations were less likely to achieve ≥50% reduction in RBC transfusions (24% [17-32%] vs. 39% [27-51%]; p=0.03). A significant correlation persisted in subjects receiving POM but not in those receiving placebo. There was also a significant correlation between U2AF1 mutations and RBC-TI in POM treated subjects compared with controls; U2AF1-mutated subjects were less likely to achieve RBC-TI (3% [1, 17%]) than U2AF1-unmutated subjects (25% [17, 34%], p=0.008). No other mutations were significantly correlated with response.
Conclusion
We found a high incidence of spliceosome mutations in persons with MPN-associated MF and RBC-transfusion-dependence. Mutation of U2AF1 correlated with response in subjects receiving POM but not in those receiving placebo. Incorporation of mutation profiling into clinical trial design may help to inform subgroups of patients that may benefit from the intervention.
Devos:Novartis: Consultancy; Celgene: Consultancy; Takeda: Consultancy. Gisslinger:Shire: Consultancy, Honoraria; Novartis: Honoraria, Research Funding; AOP Orphan Pharmaceuticals AG: Consultancy, Honoraria, Research Funding; Janssen Cilag: Consultancy, Honoraria. Kiladjian:Celgene: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Mesa:Celgene: Research Funding; UT Health San Antonio - Mays Cancer Center: Employment; Pfizer: Research Funding; Gilead: Research Funding; NS Pharma: Research Funding; CTI Biopharma: Research Funding; Incyte Corporation: Research Funding; Novartis: Consultancy; Promedior: Research Funding; Genentech: Research Funding. Ribrag:argenX: Research Funding; BMS: Consultancy, Honoraria, Other: travel; NanoString Technologies: Consultancy, Honoraria; pharmamar: Other: travel; Infinity: Consultancy, Honoraria; MSD: Honoraria; Amgen: Research Funding; Gilead: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Roche: Honoraria, Other: travel; epizyme: Consultancy, Honoraria; Incyte Corporation: Consultancy. Schiller:Celator/Jazz Pharmaceuticals: Research Funding; Pharmacyclics: Research Funding. Vannucchi:Celgene: Membership on an entity's Board of Directors or advisory committees; ITALFARMACO: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Reiser:Celgene: Employment. Zhong:Celgene: Employment. Mead:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; ARIAD: Consultancy; Bristol-Myers Squibb: Consultancy; Cell Therapeutics: Consultancy; Celgene: Research Funding; Elstar: Research Funding; Evotek: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal